APEX
Actionable, Personalized, EXpress
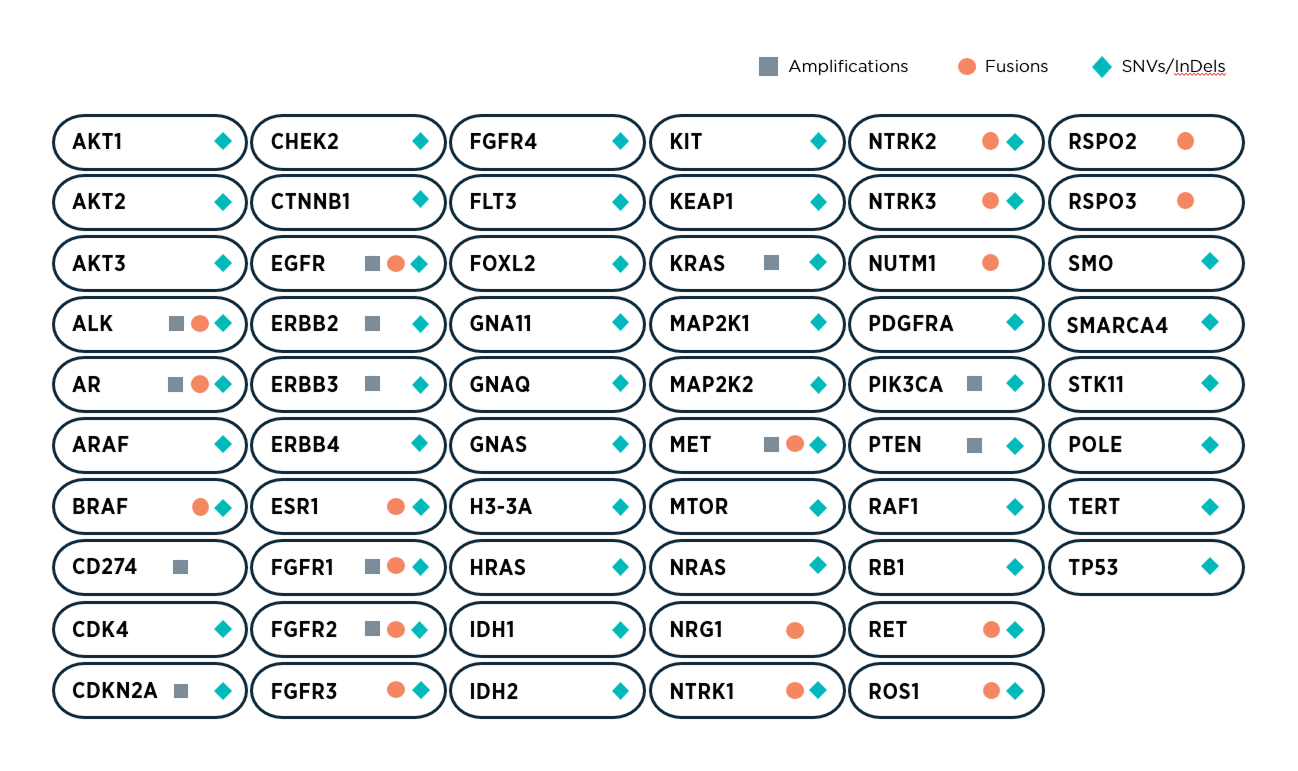
APEX Tissue is a 58-gene, targeted Next-Generation Sequencing (NGS) hotspot panel, which is specially curated for the detection of sensitizing and resistance genetic alteration to guide personalized treatment selection in key cancer types.
APEX
Actionable, Personalized, EXpress
APEX Tissue
APEX Tissue is a 58-gene, targeted Next-Generation Sequencing (NGS) hotspot panel, which is specially curated for the detection of sensitizing and resistance genetic alteration to guide personalized treatment selection in key cancer types.
Test Features
Short turnaround time of
4 working days* to allow quicker treatment initiation
Competitively priced for greater affordability and accessibility
Targeted hotspot sequencing of 58 genes specially curated based on clinical actionability
Profiling both DNA and RNA for reliable detection of genetic alterations
APEX Tissue has been clinically validated
Profiling various types of genetic alterations, such as SNVs, InDels, amplifications, and fusions, in a single test
Including treatment recommendations based on FDA approvals, NCCN and ESMO guidelines
Easy-to-read report with tier classification of variants for quick decision making
*Counting from the day the specimen is received at our laboratory in Singapore. SNVs: single nucleotide variants; InDels: insertions and deletions.
Genetic Biomarkers Relevant in Various Solid Tumors
| Cancer Type | Associated Genes |
|---|---|
| Lung | ALK, BRAF, EGFR, ERBB2, KEAP1, KRAS, MET, NRG1, NTRK1/2/3, ROS1, RET, SMARCA4, STK11 |
| Breast | AKT1, BRAF, CDK4, CDKN2A, EGFR, ERBB2, ESR1, FGFR1, MAP2K1, MTOR, NTRK1/2/3, PIK3CA, PTEN, RB1, RET |
| Colorectal | ALK, BRAF, ERBB2, FGFR1, KRAS, NRAS, NTRK1/2/3, PIK3CA,POLE, PTEN, RET, ROS1 |
| GIST | BRAF, ERBB2, FGFR1, KIT, NTRK1/2/3, PDGFRA, RET |
| Cholangiocarcinoma / Biliary / Gallbladder | BRAF, ERBB2, FGFR2, IDH1, KRAS, MET, NTRK1/2/3, PIK3CA, RET |
| Melanoma | BRAF, ERBB2, HRAS, KIT, KRAS, NRAS, MAP2K1, NTRK1/2/3, RET |
| Pancreas | ALK, BRAF, CDKN2A, EGFR, ERBB2, FGFR1/2/3, KRAS, MET, NRG1, NTRK1/2/3, RET, ROS1 |
| Thyroid | ALK, BRAF, CDKN2A, EGFR, ERBB2, FGFR2, HRAS, KIT, KRAS, NRAS, NTRK1/2/3, PIK3CA, RET, ROS1, STK11 |
| Endometrial | ALK, BRAF, CDK4, ERBB2, ESR1, FGFR2, KRAS, NTRK1/2/3, PIK3CA, POLE, PTEN, RET, SMARCA4 |
| Small Bowel | BRAF, ERBB2, KRAS, NTRK1/2/3, POLE, RET |
| Stomach / Gastric | BRAF, EGFR, ERBB2, MET, NTRK1/2/3, RET |
| Bladder / Urothelial | BRAF, ERBB2, FGFR3, NTRK1/2/3, RET |
| CNS / Brain | BRAF, ERBB2, H3-3A, IDH1, IDH2, NTRK1/2/3, RET, TERT |
Note: Not suitable for patients who have failed multiple treatments and exhausted most treatment options. A larger panel may be warranted in these patients

Sample Requirement
- Provide 10 unstained sections (5 μm thick) on uncoated/uncharged slides*
- Provide 1 matching H&E stained with tumor region marked out
- Tumor cellularity: ≥ 25%; Tumor region: ≥ 25 mm2
- Place all the slides in the slide holders provided
*Additional 5 sections are required if tumor region <25 mm2
Ordering Process for Physicians
Step 1
Request a kit online or contact our local representative
Step 2
Obtain informed consent from patient
Step 3
Request tissue slides and histopathology report from pathology laboratory
Step 4
Place all forms and slides in the box provided
Step 5
Contact our local representative to arrange for pickup and courier to Singapore laboratory
Step 6
Receive clinical report after 4 working days from kit receipt at the laboratory
What does APEX stand for?
APEX stands for Actionable, Personalized and EXpress. It is a 58-gene, targeted hotspot Next-Generation Sequencing panel specially designed for the detection of both drug-sensitizing oncogenic driver alterations and resistance mutations in a single test.
How was APEX Tissue validated?
APEX Tissue underwent a series of stringent validation processes, which encompass both analytical and clinical validations. For analytical validation, commercially available industry standards were used to validate the performance of our sequencer in specific laboratory settings. Through the public-private partnership with the Diagnostic Molecular Oncology Centre of National University Hospital, APEX Tissue was also validated using archival clinical specimens with known genetic alterations determined by orthogonal tests to establish the limits of detection for accurate reporting.
Does M Diagnostics have any accreditations?
M Diagnostics Pte. Ltd. holds the Healthcare Services Act (HCSA) 2020 License issued by the Ministry of Health (MOH) to operate as a clinical laboratory in Singapore. M Diagnostics has also been a College of American Pathologists (CAP)-accredited laboratory since 2023.
How do we ensure the consistent quality of APEX Tissue?
Quality control parameters, such as biomarkers-specific positive and negative call rates and sequencer performance indicators, are tracked and monitored monthly to ensure consistent test performance. These data constitute an important component of the audit documentations for continuous Ministry of Health Singapore licensing and the accreditations by the College of American Pathologists (CAP). The CAP Laboratory Accreditation Program is an internationally recognized laboratory inspection program, which involves a continuous process of self-inspections and external inspections by CAP-assigned inspectors to monitor and improve the laboratory’s overall quality and performance, as well as to achieve the highest standards of excellence in delivering accurate test results that would impact patient care. Besides the CAP accreditation, M Diagnostics also subscribes to the proficiency testing program offered by GenQA, an internationally recognized External Quality Assessment (EQA) provider. This ensures our laboratory proficiency through rigorous and regular external sample testing, individualized and comparative performance feedback, continual education, and alignment with international quality standards—all are fundamental to reliable and high-quality laboratory diagnostics in clinical genomics.
Can we accept formalin-fixed paraffin-embedded (FFPE) specimens that do not fulfil the minimal requirements?
Yes, we can accept FFPE specimens below the minimal requirements. Once we receive such specimens at the laboratory, our customer service team will inform you via email on the same day about the sample receival and the non-conformance of the sample acceptance criteria. Regardless, we will carry on with the extraction of nucleic acids for all cases. If the quantity or quality of the extracted nucleic acids is insufficient or suboptimal, you can then decide whether you want to continue or cancel the test. If you choose to continue the test, you should be aware that there might be a potential risk of failure or false negative result. In the test report, we will clearly indicate these precautions in terms of result interpretation so that an informed judgement can be made.
Can I use an older tissue biopsy for APEX Tissue test?
Yes, provided the tissue biopsy is not older than 1 year from the date of collection. Based on our laboratory’s experience in processing tissue samples for NGS, sequencing is more likely to fail for samples older than 1 year. However, the stability of DNA and RNA in the tissue biopsy is dependent on tissue processing techniques and storage conditions. The older tissue biopsy may also not reflect the patient’s current tumor biology and mutational profile, especially if the patient has progressed through multiple lines of treatments. Therefore, we highly recommend the submission of the latest tissue biopsy from the patient.
What are the differences between COMPASS Tissue test and APEX Tissue test?
| Product name | COMPASS Tissue | APEX Tissue |
| Number of target genes | 1021 | 58 |
| Panel design | Comprehensive genomic profiling panel | Hotspot panel |
| Technology | Hybridization capture-based Next-generation sequencing, DNA sequencing | Amplicon-based Next-generation sequencing, DNA and RNA sequencing |
| Cancer types | Solid tumors | Solid tumors |
| Specimen type | FFPE tissue | FFPE tissue |
| Types of genomic alterations covered |
|
|
| Tumor mutational burden (TMB)* | Yes | No |
| Microsatellite instability (MSI)* | Yes | No (available as a separate add-on PCR test) |
| Homologous recombination repair (HRR) genes coverage | Yes | No |
| DNA mismatch repair (MMR) genes coverage | Yes | No |
| Pharmacogenomics | Yes | No |
| Therapeutic Class Information |
|
|
| Turnaround time** | 10 working days | 4 working days |
*Clinical validation for TMB is ongoing.
**Counting from the day the specimen arrives at M Diagnostics laboratory.
MM-022-W_r00